Graham’s Law | Diffusion, Effusion, Statement, & Equation
Introduction
Graham’s law, named after the Scottish chemist Thomas Graham, is a fundamental principle in the study of gases. This law provides insights into the behaviour of gas molecules and their motion under different conditions. Graham’s work on gas diffusion and effusion significantly contributed to the development of the kinetic theory of gases, which explains the macroscopic properties of gases in terms of their microscopic behaviour.
Historical Background
Thomas Graham conducted groundbreaking research in the mid-19th century that laid the foundation for our understanding of gas diffusion and effusion. In 1829, he presented his work on the diffusion of gases at the Royal Society of Edinburgh. Graham’s experiments involved observing the rates at which different gases mixed with air through porous barriers. This led him to formulate the law that now bears his name.
Diffusion and Effusion Defined
Before delving into Graham’s law, it’s crucial to understand the concepts of diffusion and effusion.
Diffusion
Diffusion refers to the process by which particles spread from an area of higher concentration to an area of lower concentration. In the context of gases, this means that gas molecules will naturally mix and move from regions of higher pressure to regions of lower pressure.
Effusion
Effusion, on the other hand, is the process by which gas molecules pass through a tiny opening or porous barrier. This is different from diffusion, which involves the mixing of gases in open space. Effusion specifically deals with the movement of gas through a confined space or membrane.
Graham’s Law Statement
Graham’s law is formally expressed as a mathematical equation that relates the rates of effusion or diffusion of two gases. The law can be stated as follows:
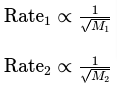
Where:
- Rate1 and Rate2 are the rates of effusion or diffusion for gases 1 and 2, respectively.
- M1 and M2 are the molar masses of gases 1 and 2, respectively.
The proportionality constant can be expressed as an equality by introducing a constant of proportionality (k):
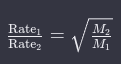
This equation encapsulates the essence of Graham’s law and is fundamental to understanding the relationship between the rates of effusion or diffusion and the molar masses of gases.
Deriving Graham’s Law Equation
To derive Graham’s law equation, let’s consider the kinetic theory of gases and the root mean square speed (rms speed) of gas molecules.
Kinetic Theory of Gases
The kinetic theory of gases postulates that gas molecules are in constant motion and collide with each other and the walls of their container. The average kinetic energy of gas molecules is directly proportional to the temperature of the gas.
Root Mean Square Speed (rms speed)
The rms speed of gas molecules is a measure of their average speed, taking into account the speeds of all individual molecules in a gas sample. It is defined by the equation:
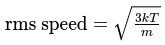
Where:
- k is the Boltzmann constant.
- T is the absolute temperature.
- m is the mass of a gas molecule.
Considering two different gases with masses m1 and m2, the ratio of their rms speeds (rms speed1/rms speed2 ) can be expressed as:

This equation forms the basis for deriving Graham’s law.
Relationship to Effusion/Diffusion
The rms speed is directly related to the rate of effusion or diffusion. The rate (Rate) can be defined as the number of moles of gas passing through a given area in a given time. For effusion, this is typically through a small opening, while for diffusion, it’s through the open space.
Rate ∝ rms speed
By combining the expressions for rms speed and the ratio of rms speeds, we arrive at Graham’s law equation:
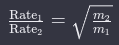
This can be further modified by substituting m1 and m2 with M1 and M2, the molar masses of the gases.
Implications and Applications
Graham’s law has several implications and applications in various scientific and industrial fields.
Predicting Gas Behavior
Graham’s law provides a tool for predicting the behavior of gases based on their molar masses. It helps in understanding how different gases disperse or effuse under specific conditions. This is crucial for designing systems where gas separation or controlled release is a consideration.
Gas Separation Techniques
In industries such as petrochemicals and natural gas processing, gas separation is a critical step. Graham’s law is employed to optimize separation processes, ensuring the efficient extraction of specific gases from complex mixtures.
Atmospheric Science
Understanding the diffusion of gases is essential in atmospheric science. Graham’s law contributes to our knowledge of how gases mix in the atmosphere, influencing phenomena such as air pollution and the dispersion of pollutants.
Effusion in Biological Systems
Gases play a vital role in biological systems, from the exchange of gases in the respiratory system to the movement of gases across cell membranes. Graham’s law helps explain and predict these processes, contributing to the understanding of physiological functions.
Pharmaceutical Industry
In pharmaceutical manufacturing, the formulation of drugs often involves gases. Graham’s law is considered when designing processes that involve the controlled release of gases, ensuring precise and efficient production.
Quality Control in Gas Mixtures
Industries that produce and use gas mixtures, such as calibration gases, rely on Graham’s law for quality control. Understanding the rates at which different components of a gas mixture diffuse or effuse is crucial for maintaining consistency in product composition.
Limitations and Considerations
While Graham’s law is a valuable tool in understanding gas behavior, it has certain limitations and considerations.
Ideal Gas Assumption
Graham’s law assumes that gases behave ideally, meaning they follow the ideal gas law. In reality, gases may deviate from ideal behavior under certain conditions, and corrections may be needed for accurate predictions.
Temperature and Pressure Effects
The law assumes constant temperature and pressure. Changes in these parameters can affect the accuracy of predictions based on Graham’s law. Corrections may be necessary for non-ideal conditions.
Molecular Size
Graham’s law does not consider the size of gas molecules. While it accurately predicts the behavior of ideal gases, real gases may exhibit deviations due to molecular size and intermolecular forces.
Conclusion
Graham’s law of diffusion and effusion is a fundamental principle in the study of gases. It provides a mathematical framework for understanding the relationship between the rates of effusion or diffusion and the molar masses of gases. Derived from the kinetic theory of gases, Graham’s law has widespread applications in various scientific and industrial fields.
As we continue to explore the behaviour of gases under different conditions, Graham’s law remains a valuable tool for predicting and optimising processes involving gas diffusion and effusion. Its principles contribute to advancements in fields such as chemistry, physics, atmospheric science, and industrial manufacturing, shaping our understanding of gas dynamics at both the macroscopic and microscopic levels.